About our Clinical Studies
Chronic urticaria
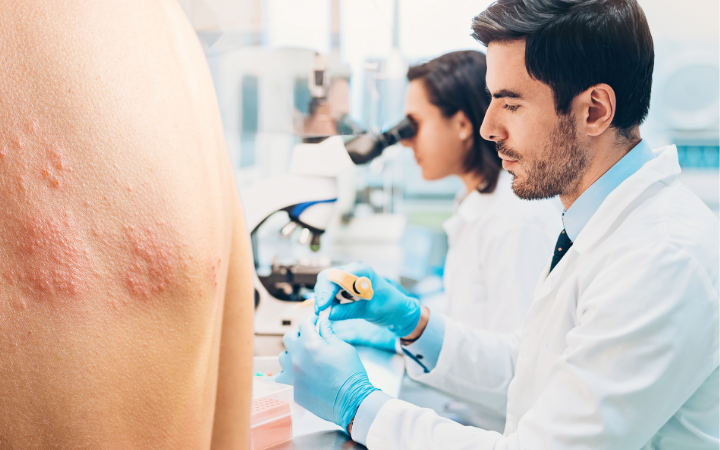
This study aims to evaluate new treatments for people living with chronic urticaria (hives).
If you qualify, you may receive professional medical care at no cost and financial compensation for your time and participation.
More information
Objective:
To evaluate the efficacy and safety of a new treatment to reduce the symptoms of chronic urticaria in adults.
Product or technique used:
Investigational biological or immunomodulatory treatment designed to control the inflammatory response that causes skin flares.
Medical Significance:
This study seeks to offer therapeutic alternatives for patients who have not responded adequately to conventional treatments, improving their quality of life.
Study Population:
Men and women over 18 years of age diagnosed with chronic urticaria that is spontaneous or persistent for more than six weeks.
Evaluation:
Participants will receive specialized medical care, periodic clinical evaluations, symptom monitoring, and laboratory tests free of charge.
Compensation:
Eligible volunteers will receive financial compensation for their time and participation, in addition to all treatments and consultations free of charge. Earn up to $2,000 by participating.
Chronic Diabetic Foot
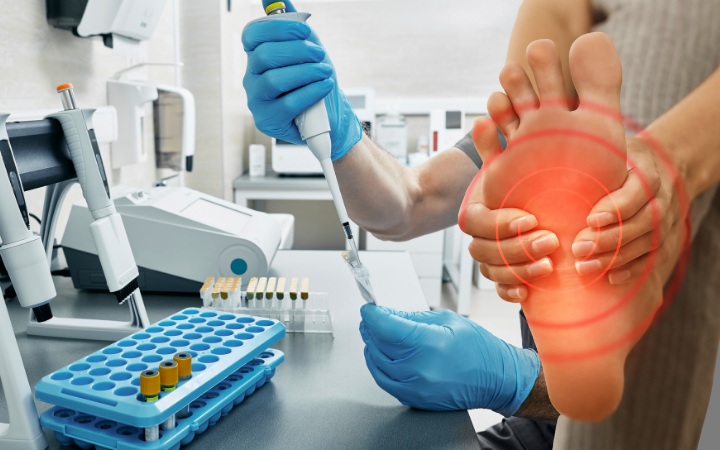
The goal of this study is to evaluate the efficacy of Derm-Maxx combined with standard of care (SOC) versus SOC alone in the healing of chronic diabetic foot ulcers.
If you qualify, you may receive professional medical care at no cost and financial compensation for your time and participation.
More information
Objective:
To evaluate the efficacy of the Derm-Maxx product combined with standard of care (SOC) versus SOC alone in the healing of chronic diabetic foot ulcers.
Product:
Derm-Maxx is a human acellular dermal matrix (ADM) that acts as a natural scaffold for skin regeneration. It does not require refrigeration, rehydrates with saline, and promotes revascularization within 7 days.
Medical Significance:
Diabetic foot ulcers cost more than $50 billion to the US healthcare system. 30% become chronic and increase the risk of amputation.
Study Population:
Adults with type 1 or II diabetes and a Wagner grade 1 or 2 ulcer. Up to 250 participants are expected to be recruited.
Evaluation:
Complete ulcer closure at 12 weeks, wound area reduction, pain reduction, and treatment safety.
Compensation:
$100 per visit (up to $1,300 per patient)
Some requirements for the studies
Remember: Bringing your medical records with you makes the process easier and ensures more accurate monitoring of your health.
Why Participate?
Sign up today and help advance medical research!
Your participation could make a real difference for people living with chronic hives and Diabetic Foot.

Medical Expertise You Can Trust

Our Miami-based medical team has extensive experience in clinical research and dermatology.
We follow strict ethical and safety standards to ensure your well-being throughout the study.

